

With the approval of deutetrabenazine and, more recently, of deucravacitinib, deuterium in drug discovery has come of age.
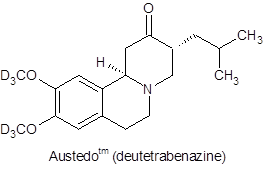
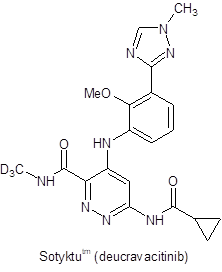
Despite the significant achievement of introducing deuterated drugs to the market, these two APIs present relatively simple challenges with regard to their deuterium content. Both introduce deuterium in the form of the deuterated methyl group - derived from methyl iodide-d3. This deuterium source is available in bulk quantities at very high enrichment. Thus, contaminants with lower levels of enrichment do not pose the problem that will arise for new deuterated APIs in which deuterium is introduced at less synthetically accessible locations. Compounds in development such as VX-561, VX-984, and DRX-065 and other targets carrying deuterated alkyl chain moieties require different strategies for deuterium incorporation. Furthermore, the addition of deuterium to an API synthesis radically restructures the cost profile of the synthesis.
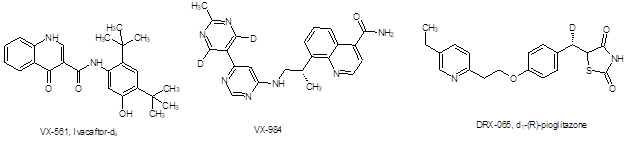
Applying deuterium chemistry to APIs requires an in-depth understanding of the field, addressing issues that include:
With many years of experience working with deuterated reagents and designing syntheses of deuterated APIs, Wakefield Chemistry Consulting can provide answers to many of these challenges. Prior to forming Wakefield Chemistry Consulting, the company principal served as the Director of Chemistry for the largest stable isotope company in the world, designing and executing many syntheses of deuterated final targets and intermediates on scales ranging form milligrams to metric tons. Since its formation, Wakefield Chemistry Consulting has provided consulting services on two deuterated API programs and continues to work in the area. Based on this experience, Wakefield Chemistry Consulting is in a unique position to assist in developing new deuterated API targets.